How To Find Mass Percent Of A Solution
13.five: Solution Concentration- Mass Per centum
- Page ID
- 47553
Learning Objectives
- Express the amount of solute in a solution in various concentration units.
To ascertain a solution precisely, nosotros need to state its concentration: how much solute is dissolved in a certain amount of solvent. Words such as dilute or concentrated are used to describe solutions that accept a little or a lot of dissolved solute, respectively, only these are relative terms with meanings that depend on various factors.
Introduction
Concentration is the measure of how much of a given substance is mixed with another substance. Solutions are said to be either dilute or concentrated. When nosotros say that vinegar is \(5\%\) acerb acrid in h2o, nosotros are giving the concentration. If we said the mixture was \(10\%\) acetic acrid, this would be more concentrated than the vinegar solution.
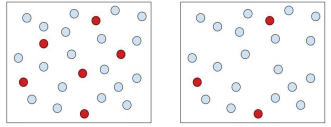
A concentrated solution is one in which in that location is a big amount of solute in a given amount of solvent. A dilute solution is ane in which there is a small amount of solute in a given amount of solvent. A dilute solution is a concentrated solution that has been, in essence, watered downward. Recall of the frozen juice containers yous purchase in the grocery store. To make juice, you take to mix the frozen juice concentrate from within these containers with three or four times the container size full of water. Therefore, you are diluting the full-bodied juice. In terms of solute and solvent, the concentrated solution has a lot of solute versus the dilute solution that would have a smaller amount of solute.
The terms "concentrated" and "dilute" provide qualitative methods of describing concentration. Although qualitative observations are necessary and have their place in every part of science, including chemistry, we have seen throughout our study of science that there is a definite demand for quantitative measurements in science. This is specially true in solution chemistry. In this section, nosotros will explore some quantitative methods of expressing solution concentration.
Mass Percent
There are several means of expressing the concentration of a solution past using a percent. The mass/mass percent (% m/chiliad) is defined as the mass of a solute divided by the mass of a solution times 100:
\[\mathrm{\% \:m/m = \dfrac{mass\: of\: solute}{mass\: of\: solution}\times100\%}\]
mass of solution = mass of solute + mass solvent
If you can measure the masses of the solute and the solution, determining the mass/mass percent is easy. Each mass must be expressed in the aforementioned units to make up one's mind the proper concentration.
Suppose that a solution was prepared by dissolving \(25.0 \: \text{one thousand}\) of sugar into \(100.0 \: \text{g}\) of h2o.
The mass of the solution is
mass of solution = 25.0g carbohydrate + 100.0g water = 125.0 g
The per centum past mass would be calculated by:
\[\text{Percent by mass} = \frac{25.0 \: \text{g carbohydrate}}{125.0 \: \text{g solution}} \times 100\% = 20.0\% \: \text{carbohydrate}\]
Example \(\PageIndex{one}\)
A saline solution with a mass of 355 g has 36.five g of NaCl dissolved in it. What is the mass/mass per centum concentration of the solution?
Solution
We tin substitute the quantities given in the equation for mass/mass percent:
\(\mathrm{\%\: m/m=\dfrac{36.5\: one thousand}{355\: g}\times100\%=ten.3\%}\)
Practise \(\PageIndex{1}\)
A dextrose (also called D-glucose, C6H12Osix) solution with a mass of 2.00 × 10ii g has 15.viii g of dextrose dissolved in it. What is the mass/mass per centum concentration of the solution?
- Reply
-
vii.90 %
Using Mass Percent in Calculations
Sometimes you may want to make upwardly a particular mass of solution of a given percent by mass and need to calculate what mass of the solute to utilise. Using mass percent as a conversion can be useful in this blazon of problem. The mass pct tin can exist expressed as a conversion factor in the form \(\frac{g \; \rm{solute}}{100 \; \rm{g solution}}\) or \(\frac{100 \; \rm chiliad solution}{one thousand\; \rm{solute}}\)
For example, if you need to make \(3000.0 \: \text{chiliad}\) of a \(5.00\%\) solution of sodium chloride, the mass of solute needs to exist determined.
Solution
Given: 3000.0 g NaCl solution
5.00% NaCl solution
Find: mass of solute = ? g NaCl
Other known quantities: five.00 g NaCl is to 100 one thousand solution
The appropriate conversion cistron (based on the given mass percent) can be used follows:
To solve for the mass of NaCl, the given mass of solution is multiplied by the conversion cistron.
\[g NaCl = iii,000.0 \cancel{one thousand \: NaCl \:solution} \times \frac{5.00 \:g \: NaCl}{100\abolish{one thousand \: NaCl \: solution}} = 150.0g \: NaCl\]
You would need to weigh out \(150 \: \text{grand}\) of \(\ce{NaCl}\) and add information technology to \(2850 \: \text{m}\) of water. Notice that it was necessary to subtract the mass of the \(\ce{NaCl}\) \(\left( 150 \: \text{g} \right)\) from the mass of solution \(\left( 3000 \: \text{g} \correct)\) to calculate the mass of the water that would demand to exist added.
Practice \(\PageIndex{1}\)
What is the amount (in g) of hydrogen peroxide (H two O ii ) needed to brand a vi.00 kg, 3.00 % (by mass) H 2 O 2 solution?
Reply
180 g H 2 O two
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Introductory_Chemistry_%28Tro%29/13:_Solutions/13.05:_Solution_Concentration-_Mass_Percent
Posted by: rettigthedidismind.blogspot.com


0 Response to "How To Find Mass Percent Of A Solution"
Post a Comment